Welcome to Our Virtual Home Dialysis Education for Health Care Professionals
How we can help
Success Stories
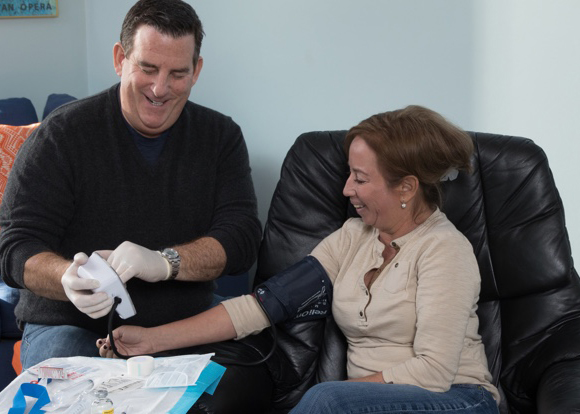
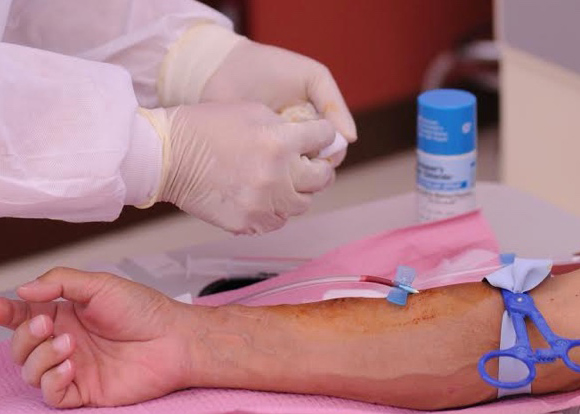
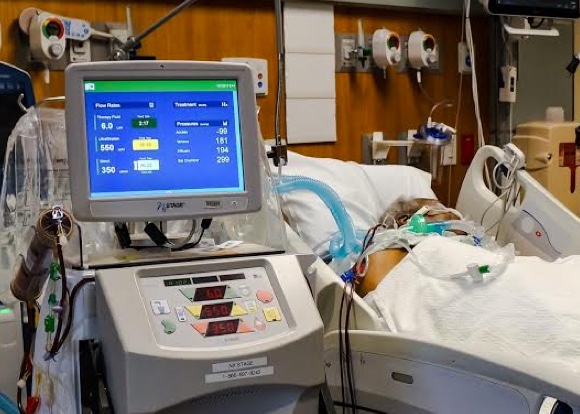
Home Hemodialysis - One decision, one life transformed
Around the world, patients like Don are thriving on NxStage® System One home hemodialysis therapy, allowing them to get back to doing the things they love.